 Predpokladá sa, že príčina ulceróznej kolitídy nejako súvisí s abnormálnymi imunologickými reakciami tela na bežne sa vyskytujúce baktérie v hrubom čreve.
Predpokladá sa, že príčina ulceróznej kolitídy nejako súvisí s abnormálnymi imunologickými reakciami tela na bežne sa vyskytujúce baktérie v hrubom čreve.
Neexistujú žiadne klinické alebo vedecké dôkazy, ktoré by podporovali teóriu, že špecializovaná diéta môže spôsobiť alebo prospieť jednotlivcom s ulceróznou kolitídou (UC). Pacienti však môžu zistiť, že niektoré potraviny zhoršujú symptómy ulceróznej kolitídy a mali by sa takýmto potravinám vyhýbať. Najčastejšími príznakmi ulceróznej kolitídy sú rektálne krvácanie, brušné kŕče a hnačka. Niektorí ľudia odporúčajú vyhýbať sa strave s vysokým obsahom vlákniny (ako je surové ovocie, zelenina, semená, orechy atď.) Okrem iných potravín, ktoré zhoršujú príznaky. Môže byť rozumné viesť si denník potravín, aby ste mohli sledovať, ktoré potraviny zhoršujú príznaky a ktoré nezhoršujú príznaky (napríklad banány, biela ryža, biely chlieb, jablkový pretlak, nevýrazné mäkké jedlá atď.). Diskutujte o svojich stravovacích potrebách so svojím ošetrujúci lekár alebo dietológ, ktorý sa špecializuje na ulceróznu kolitídu a diétu.
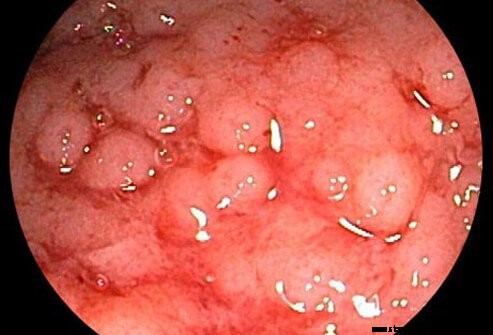 Ulcerózna kolitída sa považuje za súvisiacu s Crohnovou chorobou, ďalším chronickým zápalovým ochorením čriev (obe sú označované ako ako zápalové ochorenie čriev).
Ulcerózna kolitída sa považuje za súvisiacu s Crohnovou chorobou, ďalším chronickým zápalovým ochorením čriev (obe sú označované ako ako zápalové ochorenie čriev).
Ulcerózna kolitída je chronický zápal hrubého čreva (hrubého čreva). Hrubé črevo je časť tráviaceho systému, kde sa z nestráveného materiálu odstraňuje voda a zostávajúci odpad sa ukladá. Rektum je koniec hrubého čreva susediaci s konečníkom. U pacientov s ulceróznou kolitídou vedú vredy a zápaly vnútornej výstelky hrubého čreva k príznakom bolesti brucha, hnačky a krvácania z konečníka.
Ulcerózna kolitída úzko súvisí s ďalším stavom zápalu čriev nazývaným Crohnova choroba. Spoločne sa často označujú ako zápalové ochorenie čriev (IBD). Ulcerózna kolitída a Crohnova choroba sú chronické stavy. Crohnova choroba môže postihnúť akúkoľvek časť gastrointestinálneho traktu, vrátane všetkých vrstiev steny čreva. Nemusí sa obmedzovať len na GI trakt (postihuje pečeň, kožu, oči a kĺby). UC postihuje iba výstelku hrubého čreva (hrubého čreva). Muži a ženy sú postihnutí rovnako. Najčastejšie začínajú počas dospievania a ranej dospelosti, ale môžu začať aj v detstve a neskôr v živote.
UC sa vyskytuje na celom svete, ale najčastejšie sa vyskytuje v Spojených štátoch, Anglicku a severnej Európe. Je to bežné najmä u ľudí židovského pôvodu. Ulcerózna kolitída je zriedkavo pozorovaná vo východnej Európe, Ázii a Južnej Amerike a je zriedkavá u černošskej populácie. Z neznámych dôvodov bola nedávno pozorovaná zvýšená frekvencia tohto stavu v rozvojových krajinách.
Príbuzní prvého stupňa ľudí s ulceróznou kolitídou majú zvýšené celoživotné riziko vzniku ochorenia, ale celkové riziko zostáva malé.
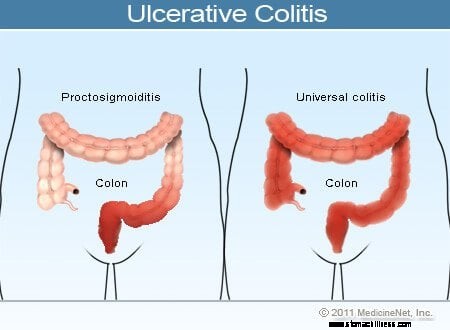 Ilustrácia ulceróznej kolitídy
Ilustrácia ulceróznej kolitídy
Bežné príznaky ulceróznej kolitídy zahŕňajú rektálne krvácanie, bolesť brucha a hnačku, ale u pacientov s týmto ochorením existuje široká škála symptómov. Variabilita symptómov odráža rozdiely v rozsahu ochorenia (množstvo zapáleného hrubého čreva a konečníka) a intenzite zápalu. Vo všeobecnosti majú pacienti so zápalom obmedzeným na konečník a krátky segment hrubého čreva susediaci s konečníkom miernejšie symptómy a lepšiu prognózu ako pacienti s rozšírenejším zápalom hrubého čreva. Rôzne typy ulceróznej kolitídy sú klasifikované podľa miesta a rozsahu zápalu:
Zatiaľ čo intenzita zápalu hrubého čreva pri ulceróznej kolitíde časom narastá a klesá, lokalizácia a rozsah ochorenia u pacienta vo všeobecnosti zostáva konštantný. Preto, keď sa u pacienta s ulceróznou proktitídou vyvinie recidíva jeho choroby, zápal sa zvyčajne obmedzí na konečník. Napriek tomu sa u malého počtu pacientov (menej ako 10 %) s ulceróznou proktitídou alebo proktosigmoiditídou môže neskôr vyvinúť rozsiahlejšia kolitída. U pacientov, ktorí mali spočiatku len ulceróznu proktitídu, sa teda môže neskôr vyvinúť ľavostranná kolitída alebo dokonca pankolitída.


Váš imunitný systém a
komplikácie UC
Príčina ulceróznej kolitídy nie je známa. K dnešnému dňu neexistuje žiadny presvedčivý dôkaz, že je spôsobená infekciou alebo je nákazlivá.
Ulcerózna kolitída pravdepodobne zahŕňa abnormálnu aktiváciu imunitného systému v črevách. Tento systém má chrániť telo pred škodlivými baktériami, vírusmi, plesňami a inými cudzími útočníkmi. Normálne sa imunitný systém aktivuje iba vtedy, keď je telo vystavené škodlivým útočníkom. U pacientov s ulceróznou kolitídou je však imunitný systém abnormálne a chronicky aktivovaný bez prítomnosti akéhokoľvek známeho útočníka. Pokračujúca abnormálna aktivácia imunitného systému spôsobuje chronický zápal a ulceráciu častí hrubého čreva. Táto náchylnosť k abnormálnej aktivácii imunitného systému je geneticky zdedená. Príbuzní prvého stupňa (bratia, sestry, deti a rodičia) pacientov s IBD majú preto väčšiu pravdepodobnosť vzniku týchto ochorení.
Uskutočnilo sa viacero štúdií s použitím skenov širokej asociácie genómu, ktoré skúmali genetickú náchylnosť na ulceróznu kolitídu. Tieto štúdie zistili, že existuje približne 30 génov, ktoré môžu zvýšiť náchylnosť na ulceróznu kolitídu vrátane génu imunoglobulínového receptora FCGR2A, 5p15, 2p16, ORMDL3, ECM1, ako aj oblastí na chromozómoch 1p36, 12q15, 7q22, 22q3R a IL2q13. V tomto ranom bode výskumu stále nie je jasné, ako budú tieto genetické asociácie aplikované na liečbu choroby, ale môžu mať budúce dôsledky pre pochopenie patogenézy a vytváranie nových liečebných postupov.
Diagnózu ulceróznej kolitídy naznačujú symptómy bolesti brucha, rektálneho krvácania a hnačky. Keďže neexistuje zlatý štandard na diagnostiku, konečná diagnóza sa opiera o kombináciu symptómov, vzhľad výstelky hrubého čreva v čase endoskopie, histologické znaky biopsií výstelky hrubého čreva a štúdie stolice na vylúčenie prítomnosti infekčných látky, ktoré môžu spôsobiť zápal.
Znalosť rozsahu a závažnosti kolitídy je dôležitá pri výbere medzi možnosťami liečby.
Niektoré novšie diagnostické modality zahŕňajú videokapsulovú endoskopiu a CT/MRI enterografiu. Videokapsulová endoskopia (VCE) môže byť užitočná na detekciu ochorenia tenkého čreva u pacientov s diagnózou UC s atypickými znakmi, u ktorých môže byť podozrenie, že skutočne majú Crohnovu chorobu. S VCE pacienti prehĺtajú kapsulu, ktorá obsahuje kameru, ktorá fotí počas cesty cez črevá a odosiela snímky bezdrôtovo do rekordéra. Obrázky sa potom skontrolujú. VCE v štúdii v roku 2007 potvrdila prítomnosť ochorenia tenkého čreva asi u 15 % pacientov s ulceróznou kolitídou s atypickými znakmi alebo neklasifikovaným zápalovým ochorením čriev, čím zmenila diagnózu na Crohnovu chorobu (ktorá sa neobmedzuje len na hrubé črevo ako napr. UC). Toto môže byť užitočná diagnostická metóda v tejto špecifickej populácii pacientov.
CT a MRI enterografia sú zobrazovacie techniky, ktoré používajú perorálne tekuté kontrastné látky pozostávajúce z roztokov PEG alebo nízkej koncentrácie bária na poskytnutie adekvátnejšieho roztiahnutia hrubého čreva a tenkého čreva. Uvádza sa, že tieto techniky sú lepšie ako štandardné zobrazovacie techniky pri hodnotení patológie tenkého čreva u pacientov s Crohnovou chorobou. Ukázalo sa tiež, že poskytujú primerané odhady závažnosti ochorenia pri ulceróznej kolitíde (s určitými podhodnoteniami a nadhodnoteniami).
Pacienti s ulceróznou kolitídou obmedzenou na konečník (proktitída) alebo kolitídou obmedzenou na koniec ľavého hrubého čreva (proktosigmoiditída) sa zvyčajne majú celkom dobre. Krátke pravidelné liečby pomocou perorálnych liekov alebo klystírov môžu byť dostatočné. Závažné komplikácie sú u týchto pacientov zriedkavé. U pacientov s rozsiahlejším ochorením môže strata krvi zo zapálených čriev viesť k anémii a môže vyžadovať liečbu doplnkami železa alebo dokonca krvné transfúzie. Zriedkavo sa hrubé črevo môže akútne roztiahnuť na veľkú veľkosť, keď sa zápal stane veľmi závažným. Tento stav sa nazýva toxický megakolón. Pacienti s toxickým megakolónom sú extrémne chorí s horúčkou, bolesťami brucha a distenziou, dehydratáciou a podvýživou. Pokiaľ sa pacient rýchlo nezlepší pomocou liekov, je zvyčajne potrebný chirurgický zákrok, aby sa predišlo ruptúre hrubého čreva.
V publikovanej škandinávskej štúdii s viac ako 500 pacientmi s ulceróznou kolitídou sledovaných až 10 rokov po diagnóze sa zistilo, že ich úmrtnosť sa nelíši od bežnej populácie. Tiež kumulatívna potreba kolektómie po 10 rokoch bola 9,8 %, takmer 50 % pacientov bolo bez relapsu v posledných piatich rokoch štúdie a iba 20 % pacientov s proktitídou alebo ľavostranným ochorením progredovalo do pankolitídy.
Rakovina hrubého čreva je známou komplikáciou chronickej ulceróznej kolitídy. Riziko rakoviny začína stúpať po ôsmich až desiatich rokoch kolitídy. Pacienti s iba ulceróznou proktitídou pravdepodobne nemajú zvýšené riziko rakoviny hrubého čreva v porovnaní s bežnou populáciou. U pacientov s aktívnou pankolitídou (zahŕňajúcou celé hrubé črevo) počas 10 rokov alebo dlhšie je riziko rakoviny hrubého čreva zvýšené v porovnaní s bežnou populáciou. U pacientov s kolitídou obmedzenou na ľavú stranu hrubého čreva je riziko rakoviny hrubého čreva zvýšené, ale nie také vysoké ako u pacientov s chronickou pankolitídou.
Pacienti s vyšším rizikom rakoviny sú pacienti s pozitívnou rodinnou anamnézou rakoviny hrubého čreva, dlhým trvaním kolitídy, rozsiahlym postihnutím hrubého čreva a primárnou sklerotizujúcou cholangitídou (PSC), ďalšou komplikáciou ulceróznej kolitídy.
Keďže tieto rakoviny majú priaznivejší výsledok, keď sú diagnostikované a liečené v skoršom štádiu, po ôsmich rokoch známeho rozsiahleho ochorenia sa môže odporučiť každoročné vyšetrenie hrubého čreva. Počas týchto vyšetrení sa môžu odobrať vzorky tkaniva (biopsie) na hľadanie prekanceróznych zmien vo výstelkových bunkách hrubého čreva. Keď sa zistia prekancerózne zmeny, môže byť potrebné odstránenie hrubého čreva, aby sa zabránilo rakovine hrubého čreva.
Komplikácie ulceróznej kolitídy môžu postihnúť aj iné časti tela.
Na liečbu ulceróznej kolitídy sa používajú lieky aj chirurgický zákrok. Operácia je však vyhradená pre pacientov s ťažkým zápalom a život ohrozujúcimi komplikáciami. Neexistuje žiadny liek, ktorý by dokázal vyliečiť ulceróznu kolitídu. U pacientov s ulceróznou kolitídou sa typicky vyskytnú obdobia recidívy (zhoršenie zápalu), po ktorých nasledujú obdobia remisie (vymiznutie zápalu) trvajúce mesiace až roky. Počas relapsov sa príznaky bolesti brucha, hnačky a krvácania z konečníka zhoršujú. Počas remisií tieto príznaky ustupujú. Remisie sa zvyčajne vyskytujú v dôsledku liečby liekmi alebo chirurgickým zákrokom, ale občas sa vyskytujú spontánne, to znamená bez akejkoľvek liečby.
Keďže ulcerózna kolitída sa nedá vyliečiť liekmi, cieľom liečby liekmi je 1) vyvolať remisie, 2) udržať remisie, 3) minimalizovať vedľajšie účinky liečby, 4) zlepšiť kvalitu života a 5) minimalizovať riziko rakoviny . Liečba ulceróznej kolitídy pomocou liekov je podobná, aj keď nie vždy identická, ako liečba Crohnovej choroby.
Lieky na liečbu ulceróznej kolitídy zahŕňajú 1) protizápalové látky, ako sú zlúčeniny 5-ASA, systémové kortikosteroidy, lokálne kortikosteroidy a 2) imunomodulátory.
Protizápalové lieky, ktoré znižujú zápal čriev, sú analogické s liekmi na artritídu, ktoré znižujú zápal kĺbov (artritída). Protizápalové lieky, ktoré sa používajú pri liečbe ulceróznej kolitídy, sú:
Imunomodulátory sú lieky, ktoré potláčajú imunitný systém tela buď znížením počtu buniek, ktoré sú zodpovedné za imunitu, alebo zasahovaním do proteínov, ktoré sú dôležité pri podpore zápalu. Imunomodulátory sa čoraz viac stávajú dôležitou liečbou pre pacientov s ťažkou ulceróznou kolitídou, ktorí nereagujú adekvátne na protizápalové látky. Príklady imunomodulátorov zahŕňajú 6-merkaptopurín (6-MP), azatioprín (Imuran), metotrexát (Rheumatrex, Trexall), cyklosporín (Gengraf, Neoral).
Dlho sa pozorovalo, že riziko ulceróznej kolitídy sa zdá byť vyššie u nefajčiarov a u bývalých fajčiarov. Za určitých okolností sa pacienti pri liečbe nikotínom zlepšujú.
5-ASA (kyselina 5-aminosalicylová), tiež nazývaná mesalamín, je chemicky podobná aspirínu. Aspirín (kyselina acetylsalicylová) sa už mnoho rokov používa na liečbu artritídy, burzitídy a tendinitídy (stavov zápalu tkaniva). Aspirín však nie je účinný pri liečbe ulceróznej kolitídy. Na druhej strane môže byť 5-ASA účinný pri liečbe ulceróznej kolitídy, ak sa liek môže podávať priamo (topicky) na zapálenú výstelku hrubého čreva. Napríklad klystír Rowasa je roztok 5-ASA, ktorý je účinný pri liečbe zápalu v konečníku av jeho blízkosti (ulcerózna proktitída a ulcerózna proktosigmoiditída). Roztok klystíru však nemôže dosiahnuť dostatočne vysokú úroveň na liečbu zápalu v hornej časti hrubého čreva. Preto sa u väčšiny pacientov s ulceróznou kolitídou musí 5-ASA užívať perorálne. Keď sa však čistá 5-ASA užíva perorálne, žalúdok a horná časť tenkého čreva absorbujú väčšinu lieku skôr, ako sa dostane do hrubého čreva. Preto, aby bol 5-ASA účinný ako perorálny prostriedok na ulceróznu kolitídu, musí byť chemicky modifikovaný, aby unikol absorpcii žalúdkom a hornými črevmi. Tieto modifikované zlúčeniny 5-ASA sú sulfasalazín (Azulfidín), mesalamín (Pentasa, Rowasa, Asacol, Lialda, Apriso) a olsalazín (Dipentum).
Sulfasalazín (azulfidín) sa už mnoho rokov úspešne používa na navodenie remisie u pacientov s miernou až stredne ťažkou ulceróznou kolitídou. Vyvolanie remisie znamená zníženie zápalu čriev a zmiernenie symptómov bolesti brucha, hnačky a krvácania z konečníka. Sulfasalazín sa tiež používa dlhší čas na udržanie remisií.
Sulfasalazín pozostáva z molekuly 5-ASA chemicky spojenej s molekulou sulfapyridínu. (Sulfapyridín je sulfa antibiotikum). Spojenie týchto dvoch molekúl zabraňuje absorpcii v žalúdku a horných črevách pred dosiahnutím hrubého čreva. Keď sulfasalazín dosiahne hrubé črevo, baktérie v hrubom čreve prerušia spojenie medzi týmito dvoma molekulami. Po odlúčení od 5-ASA sa sulfapyridín absorbuje do tela a potom sa vylučuje močom. Väčšina aktívneho liečiva 5-ASA však zostáva v hrubom čreve na liečbu kolitídy.
Väčšina vedľajších účinkov sulfasalazínu je spôsobená molekulou sulfapyridínu. Tieto vedľajšie účinky zahŕňajú nevoľnosť, pálenie záhy, bolesť hlavy, anémiu, kožné vyrážky a v zriedkavých prípadoch hepatitídu a zápal obličiek. U mužov môže sulfasalazín znížiť počet spermií. Zníženie počtu spermií je reverzibilné a počet sa zvyčajne vráti do normálu po vysadení sulfasalazínu alebo po zmene na inú zlúčeninu 5-ASA.
Výhody sulfasalazínu vo všeobecnosti závisia od dávky. Preto môžu byť na vyvolanie remisie potrebné vysoké dávky sulfasalazínu. Niektorí pacienti nemôžu tolerovať vysoké dávky kvôli nevoľnosti a žalúdočným ťažkostiam. Na minimalizáciu žalúdočných ťažkostí sa sulfasalazín vo všeobecnosti užíva po jedle alebo s jedlom. Pre niektorých pacientov je jednoduchšie užívať Azulfidín-EN (enterosolventná forma sulfasalazínu). Enterosolventný povlak pomáha znižovať žalúdočnú nevoľnosť. Novšie zlúčeniny 5-ASA neobsahujú sulfapyridínovú zložku a majú menej vedľajších účinkov ako sulfasalazín.
Asacol je tableta pozostávajúca zo zlúčeniny 5-ASA, mesalamínu, obklopená povlakom z akrylovej živice. (Asacol neobsahuje sulfanu.) Živicový povlak zabraňuje absorpcii 5-ASA pri prechode žalúdkom a tenkým črevom. Keď tableta dosiahne terminálny ileum a hrubé črevo, živicový obal sa rozpustí, čím sa uvoľní 5-ASA do hrubého čreva.
Asacol je účinný pri navodení remisie u pacientov s miernou až stredne ťažkou ulceróznou kolitídou. Je tiež účinný, keď sa používa dlhší čas na udržanie remisie. Odporúčaná dávka Asacolu na vyvolanie remisie sú dve 400 mg tablety trikrát denne (spolu 2,4 gramu denne). Two tablets of Asacol twice daily (1.6 grams a day) is recommended for maintaining remission. Occasionally, the maintenance dose is higher.
As with Azulfidine, the benefits of Asacol are dose-related. If patients do not respond to 2.4 grams a day of Asacol, the dose frequently is increased to 3.6 grams a day (and sometimes even higher) to induce remission. If patients fail to respond to the higher doses of Asacol, then alternatives, such as corticosteroids, are considered.
Lialda (mesalamine multi matrix, MMX) is an extended release formulation. It is a 5-ASA medication within an inert matrix that is surrounded by a coating. When the capsule reaches the distal ileum, the outer coating (the capsule) dissolves. The intestinal fluid then is absorbed into the matrix forming a gel-like substance which prolongs the contact of the medication with the colonic wall as the mesalamine slowly separates from the matrix. This extended release formulation allows for higher doses to be taken less frequently throughout the day and might and improve compliance.
The most common side effects experienced while taking Lialda are flatulence, abdominal pain, headache, nausea, and dyspepsia.
Apriso is another formulation of 5-ASA that consists of extended-release mesalamine granules encased in microcrystalline cellulose within a capsule. Dissolution of the capsule occurs in the distal ileum, and, since the granules are encased in the cellulose and only slowly separates from the cellulose, there is prolonged delivery of medication as the cellulose and mesalamine travel through the colon.
The most common side effects of this medication are headache, diarrhea, abdominal pain, nausea, nasopharyngitis, influenza-like illness, and sinusitis.
Pentasa is a capsule consisting of the 5-ASA compound mesalamine inside controlled-release spheres. Like Asacol, it is sulfa free. As the capsule travels down the intestines, the 5-ASA inside the spheres is slowly released into the intestines. Unlike Asacol, the mesalamine in Pentasa is released into the small intestine as well as the colon. Therefore, Pentasa can be effective in treating inflammation in the small intestine and the colon. Pentasa is currently the most logical 5-ASA compound for treating mild to moderate Crohn's disease involving the small intestine. Pentasa also is used to induce remission and maintain remission among patients with mild to moderate ulcerative colitis.
Olsalazine (Dipentum) consists of two 5-ASA molecules linked together. It is sulfa free. The linked 5-ASA molecules travel through the stomach and the small intestine unabsorbed. When the drug reaches the terminal ileum and the colon, the normal bacteria in the intestine break the linkage and release the active drug into the colon and the terminal ileum. Olsalazine has been used in treating ulcerative colitis and in maintaining remissions. A side effect unique to olsalazine is secretory diarrhea (diarrhea resulting from excessive production of fluid in the intestines). This condition occurs in some patients, and the diarrhea sometimes can be severe.
Balsalazide (Colazal) is a capsule in which the 5-ASA is linked by a chemical bond to another molecule that is inert (without effect on the intestine) and prevents the 5-ASA from being absorbed. This drug is able to travel through the intestine unchanged until it reaches the end of the small bowel (terminal ileum) and colon. There, intestinal bacteria break apart the 5-ASA and the inert molecule, releasing the 5-ASA. Because intestinal bacteria are most abundant in the terminal ileum and colon, Colazal is used to treat inflammation predominantly localized to the colon.
More clinical trials are needed to compare the effectiveness of Colazal to the other mesalamine compounds such as Asacol in treating ulcerative colitis. Therefore in the United States, choosing which 5-ASA compound has to be individualized. Some doctors prescribe Colazal for patients who cannot tolerate or fail to respond to Asacol. Others prescribe Colazal for patients with predominantly left sided colitis, since some studies seem to indicate that Colazal is effective in treating left sided colitis.
The sulfa-free 5-ASA compounds have fewer side effects than sulfasalazine and also do not impair male fertility. In general, they are safe medications for long-term use and are well-tolerated.
Patients allergic to aspirin should avoid 5-ASA compounds because they are chemically similar to aspirin.
Rare kidney inflammation has been reported with the use of 5-ASA compounds. These compounds should be used with caution in patients with known kidney disease. It also is recommended that blood tests of kidney function be obtained before starting and periodically during treatment.
Rare instances of acute worsening of diarrhea, cramps, and abdominal pain may occur which is at times may be accompanied by fever, rash, and malaise. This reaction is believed to represent an allergy to the 5-ASA compound.
Rowasa is the 5-ASA compound mesalamine in enema form and is effective in ulcerative proctitis and ulcerative proctosigmoiditis (two conditions where active 5-ASA drugs taken as enemas can easily reach the inflamed tissues directly). Each Rowasa enema contains 4 grams of mesalamine in 60 cc of fluid. The enema usually is administered at bedtime, and patients are encouraged to retain the enema through the night.
The enema contains sulfite and should not be used by patients with sulfite allergy. Otherwise, Rowasa enemas are safe and well-tolerated.
Rowasa also comes in suppository form for treating limited proctitis. Each suppository contains 500 mg of mesalamine and usually is administered twice daily.
While some patients improve within several days of starting Rowasa, the usual course of treatment is three to six weeks. Some patients may need even longer courses of treatment for optimal benefit. In patients who do not respond to Rowasa, oral 5-ASA compounds (such as Asacol) can be added. Some studies have reported increased effectiveness in treating ulcerative proctitis and proctosigmoiditis by combining oral 5-ASA compounds with Rowasa enemas. Oral 5-ASA compounds also are used to maintain remission in ulcerative proctitis and proctosigmoiditis.
Another alternative for patients who fail to respond to Rowasa or who cannot use Rowasa is cortisone enemas (Cortenema). Cortisone is a potent anti-inflammatory agent. Oral corticosteroids are systemic drugs with serious and predictable long-term side effects. Cortenema is a topical corticosteroid that has less absorption into the body than oral corticosteroids, and, therefore, it has fewer and less severe side effects.
Corticosteroids (Prednisone, prednisolone, hydrocortisone, etc.) have been used for many years in the treatment of patients with moderate to severe Crohn's disease and ulcerative colitis or who fail to respond to optimal doses of 5-ASA compounds. Unlike the 5-ASA compounds, corticosteroids do not require direct contact with the inflamed intestinal tissues to be effective. Oral corticosteroids are potent anti-inflammatory agents. After absorption, corticosteroids exert prompt anti-inflammatory action throughout the body. Consequently, they are used in treating Crohn's enteritis, ileitis, and ileocolitis, as well as ulcerative and Crohn's colitis. In critically ill patients, intravenous corticosteroids (such as hydrocortisone) can be given in the hospital.
Corticosteroids are faster acting than the 5-ASA compounds. Patients frequently experience improvement in their symptoms within days of starting corticosteroids. Corticosteroids, however, do not appear to be useful in maintaining remissions in ulcerative colitis.
Side effects of corticosteroids depend on the dose and duration of use. Short courses of prednisone, for example, usually are well tolerated with few and mild side effects. Long term, high doses of corticosteroids usually produce predictable and potentially serious side effects. Common side effects include rounding of the face (moon face), acne, increased body hair, diabetes, weight gain, high blood pressure, cataracts, glaucoma, increased susceptibility to infections, muscle weakness, depression, insomnia, mood swings, personality changes, irritability, and thinning of the bones (osteoporosis) with an accompanying increased risk of compression fractures of the spine. Children on corticosteroids can experience stunted growth.
The most serious complication from long term corticosteroid use is aseptic necrosis of the hip joints. Aseptic necrosis means death of bone tissue. It is a painful condition that can ultimately lead to the need for surgical replacement of the hips. Aseptic necrosis also has been reported in knee joints. It is unknown how corticosteroids cause aseptic necrosis. Patients on corticosteroids who develop pain in the hips or knees should report the pain to their doctors promptly. Early diagnosis of aseptic necrosis with cessation of corticosteroids has been reported in some patients to decrease the severity of the condition and possibly help avoid hip replacement.
Prolonged use of corticosteroids can depress the ability of the body's adrenal glands to produce cortisol (a natural corticosteroid necessary for proper functioning of the body). Abruptly discontinuing corticosteroids can cause symptoms due to a lack of natural cortisol (a condition called adrenal insufficiency). Symptoms of adrenal insufficiency include nausea, vomiting, and even shock. Withdrawing corticosteroids too quickly also can produce symptoms of joint aches, fever, and malaise. Therefore, corticosteroids need to be gradually reduced rather than abruptly stopped.
Even after the corticosteroids are discontinued, the adrenal glands' ability to produce cortisol can remain depressed for months to two years. The depressed adrenal glands may not be able to produce enough cortisol to help the body handle stress such as accidents, surgery, and infections. These patients will need treatment with corticosteroids (prednisone, hydrocortisone, etc.) during stressful situations to avoid developing adrenal insufficiency.
Because corticosteroids are not useful in maintaining remission in ulcerative colitis and Crohn's disease and because they have predictable and potentially serious side effects, these drugs should be used for the shortest possible length of time.
Once the decision is made to use oral corticosteroids, treatment usually is initiated with prednisone, 40-60 mg daily. The majority of patients with ulcerative colitis respond with an improvement in symptoms. Once symptoms improve, prednisone is reduced by 5-10 mg per week until the dose of 20 mg per day is reached. The dose then is tapered at a slower rate until the prednisone ultimately is discontinued. Gradually reducing corticosteroids not only minimizes the symptoms of adrenal insufficiency, it also reduces the chances of abrupt relapse of the colitis.
Many doctors use 5-ASA compounds at the same time as corticosteroids. In patients who achieve remission with systemic corticosteroids, 5-ASA compounds such as Asacol are often continued to maintain remissions.
In patients whose symptoms return during reduction of the dose of corticosteroid, the dose of corticosteroids is increased slightly to control the symptoms. Once the symptoms are under control, the reduction can resume at a slower pace. Some patients become corticosteroid dependent. These patients consistently develop symptoms of colitis whenever the corticosteroid dose reaches below a certain level. In patients who are corticosteroid dependent or who are unresponsive to corticosteroids, other anti-inflammatory medications, immunomodulator medications or surgery are considered.
The management of patients who are corticosteroid dependent or patients with severe disease which responds poorly to medications is complex. Doctors who are experienced in treating inflammatory bowel disease and in using the immunomodulators should evaluate these patients.
Long-term use of corticosteroids such as prednisolone or prednisone can cause osteoporosis . Corticosteroids cause decreased calcium absorption from the intestines and increased loss of calcium from the kidneys and bones. Increasing dietary calcium intake is important but alone cannot halt corticosteroid-induced bone loss. Management of patients on long term corticosteroids should include:
Oral budesonide (Entocort EC) is a topically acting corticosteroid which was been shown to be effective in Crohn's disease, and in enema formulation for left-sided ulcerative colitis with fewer side effects than oral steroids. In a recent meta-analysis, however, it was found to be significantly less likely to induce clinical remission in patients with ulcerative colitis than oral mesalamine after 8 weeks of therapy. Therefore, use of this medication is not recommended at this time to treat flares of ulcerative colitis.
Golimumab is an injectable man-made protein that binds to tumor necrosis factor alpha in the body, and blocks the effects of tumor necrosis factor alfa in patients with ulcerative colitis. Golimumab is injected under the skin, and injection sites should be rotated. Golimumab interacts with several drugs and has several side effects. Consult with your prescribing physician or pharmacist to discuss these potential drug interactions and side effects.
Immunomodulators are medications that weaken the body's immune system. The immune system is composed of immune cells and the proteins that these cells produce. These cells and proteins serve to defend the body against harmful bacteria, viruses, fungi, and other foreign invaders. Activation of the immune system causes inflammation within the tissues where the activation occurs. (Inflammation is, in fact, an important mechanism to defend the body used by the immune system.) Normally, the immune system is activated only when the body is exposed to harmful invaders. In patients with Crohn's disease and ulcerative colitis, however, the immune system is abnormally and chronically activated in the absence of any known invader. Immunomodulators decrease tissue inflammation by reducing the population of immune cells and/or by interfering with their production of proteins that promote immune activation and inflammation. Generally, the benefits of controlling moderate to severe ulcerative colitis outweigh the risks of infection due to weakened immunity. Examples of immunomodulators include azathioprine (Imuran), 6-mercaptopurine (6-MP, Purinethol), cyclosporine (Sandimmune), and methotrexate (Rheumatrex, Trexall).
Azathioprine and 6-mercaptopurine (6-MP) are medications that weaken the body's immunity by reducing the population of a class of immune cells called lymphocytes. Azathioprine and 6-MP are related chemically. Specifically, azathioprine is converted into 6-MP inside the body. In high doses, these two drugs have been useful in preventing rejection of transplanted organs and in treating leukemia. In low doses, they have been used for many years to treat patients with moderate to severe Crohn's disease and ulcerative colitis.
Azathioprine and 6-MP are increasingly recognized by doctors as valuable drugs in treating Crohn's disease and ulcerative colitis. Some 70% of patients with moderate to severe disease will benefit from these drugs. Because of the slow onset of action and the potential for side effects, however, 6-MP and azathioprine are used mainly in the following situations:
When azathioprine and 6-MP are added to corticosteroids in the treatment of ulcerative colitis patients who do not respond to corticosteroids alone, there may be an improved response or smaller doses and shorter courses of corticosteroids may be effective. Some patients can discontinue corticosteroids altogether without experiencing relapses. The ability to reduce corticosteroid requirements has earned 6-MP and azathioprine their reputation as "steroid-sparing" medications.
In patients with severe ulcerative colitis who suffer frequent relapses, 5-ASA may not be sufficient, and more potent azathioprine and 6-MP will be necessary to maintain remissions. In the doses used for treating ulcerative colitis and Crohn's disease, the long-term side effects of azathioprine and 6-MP are less serious than long-term oral corticosteroids or repeated courses of oral corticosteroids.
Side effects of 6-MP and azathioprine include increased vulnerability to infections, inflammation of the liver (hepatitis) and pancreas, (pancreatitis), and bone marrow toxicity (interfering with the formation of cells that circulate in the blood).
The goal of treatment with 6-MP and azathioprine is to weaken the body's immune system in order to decrease the intensity of inflammation in the intestines; however, weakening the immune system increases the patient's vulnerability to infections. For example, in a group of patients with severe Crohn's disease unresponsive to standard doses of azathioprine, raising the dose of azathioprine helped to control the disease, but two patients developed cytomegalovirus (CMV) infection. CMV usually infects individuals with weakened immune systems such as patients with AIDS or cancer, especially if they are receiving chemotherapy, which further weakens the immune system.
Azathioprine and 6-MP-induced inflammation of the liver (hepatitis) and pancreas (pancreatitis) are rare. Pancreatitis typically causes severe abdominal pain and sometimes vomiting. Pancreatitis due to 6-MP or azathioprine occurs in a small percentage of patients, usually during the first several weeks of treatment. Patients who develop pancreatitis should not receive either of these two medications again.
Azathioprine and 6-MP also suppress the bone marrow. The bone marrow is where red blood cells, white blood cells, and platelets are made. Actually, a slight reduction in the white blood cell count during treatment is desirable since it indicates that the dose of 6-MP or azathioprine is high enough to have an effect; however, excessively low red or white blood cell counts indicates bone marrow toxicity. Therefore, patients on 6-MP and azathioprine should have periodic blood counts (usually every two weeks initially and then every 3 months during maintenance) to monitor the effect of the drugs on their bone marrow.
6-MP can reduce the sperm count in men. When the partners of male patients on 6-MP conceive, there is a higher incidence of miscarriages and vaginal bleeding. There also are respiratory difficulties in the newborn. Therefore, it is recommended that whenever feasible, male patients should stop 6-MP and azathioprine for three months before attempting to conceive.
Patients on long-term, high dose azathioprine to prevent rejection of the kidney after kidney transplantation have an increased risk of developing lymphoma, a malignant disease of lymphatic cells. There is no evidence at present that long term use of azathioprine and 6-MP in the low doses used in IBD increases the risk for lymphoma, leukemia or other malignancies.
One problem with 6-MP and azathioprine is their slow onset of action. Typically, full benefit of these drugs is not realized for three months or longer. During this time, corticosteroids frequently have to be maintained at high levels to control inflammation.
The reason for this slow onset of action is partly due to the way doctors prescribe 6-MP. Typically, 6-MP is started at a dose of 50 mg daily. The blood count is then checked two weeks later. If the white blood cell count (specifically the lymphocyte count) is not reduced, the dose is increased. This cautious, stepwise approach helps prevent severe bone marrow and liver toxicity, but also delays benefit from the drug.
Studies have shown that giving higher doses of 6-MP early can speed up the benefit of 6-MP without increased toxicity in most patients, but some patients do develop severe bone marrow toxicity. Therefore, the dose of 6-MP has to be individualized. Scientists now believe that an individual's vulnerability to 6-MP toxicity is genetically inherited. Blood tests can be performed to identify those individuals with increased vulnerability to 6-MP toxicity. In these individuals, lower initial doses can be used. Blood tests can also be performed to measure the levels of certain by-products of 6-MP. The levels of these by-products in the blood help doctors more quickly determine whether the dose of 6-MP is right for the patient.
Azathioprine is converted into 6-MP in the body, and 6-MP then is partially converted into other chemicals by an enzyme called thiopurine methyltransferase (TPMT). These chemicals then are eliminated from the body. The activity of TPMT that determines the ability to convert 6-MP into other chemicals is genetically determined, and approximately 10% of the population in the United States has reduced or absent TPMT activity. In this 10% of patients, 6-MP and another related chemical (6-thioguanine or 6-TG) accumulate and are toxic to the bone marrow where blood cells are produced. Thus, when given normal doses of azathioprine or 6-MP, these patients with reduced or absent TPMT activity can develop seriously low white blood cell counts for prolonged periods of time, exposing them to serious life-threatening infections.
The Food and Drug Administration now recommends that doctors check TPMT levels prior to starting treatment with azathioprine or 6-MP. Patients found to have genes associated with reduced or absent TPMT activity are treated with alternative medications or are prescribed substantially lower than normal doses of 6-MP or azathioprine.
A word of caution is in order, however. Having normal TPMT genes is no guarantee against azathioprine or 6-MP toxicity. Rarely, a patient with normal TPMT genes can develop severe toxicity in the bone marrow and a low white blood cell count even with normal doses of 6-MP or azathioprine. In addition, with normal levels of TPMT activity, liver toxicity, another toxic effect of 6-MP, can still occur. Therefore, all patients taking 6-MP or azathioprine (regardless of TPMT genetics) have to be closely monitored by a doctor who will order periodic blood counts, and liver enzyme tests for as long as the medication is taken.
Another cautionary note:allopurinol (Zyloprim), used in treating high blood uric acids levels and gout, can induce bone marrow toxicity when used together with azathioprine or 6-MP. This occurs because allopurinol reduces TMPT activity. The combination of genetically-reduced TMPT activity and further reduction of TMPT activity by the allopurinol increases greatly the risk of bone marrow toxicity.
In addition to monitoring blood cell counts and liver tests, doctors also may measure blood levels of the chemicals that are formed from 6-MP (6-MP metabolites), which can be helpful in several situations such as:
Patients have been maintained on 6-MP or azathioprine for years without any important long-term side effects. Their doctors, however, should closely monitor their patients on long-term 6-MP. There is data suggesting that patients on long-term maintenance with 6-MP or azathioprine fare better than those who stop these medications. Those who stop 6-MP or azathioprine are more likely to experience relapses, more likely to need corticosteroids or undergo surgery.
Methotrexate (Rheumatrex, Trexall) is an immunomodulator and anti-inflammatory medication. Methotrexate has been used for many years in the treatment of severe rheumatoid arthritis and psoriasis. It has been helpful in treating patients with moderate to severe Crohn's disease who are either not responding to 6-MP and azathioprine or are intolerant of these two medications. Methotrexate also may be effective in patients with moderate to severe ulcerative colitis who are not responding to corticosteroids or 6-MP and azathioprine. It can be given orally or by weekly injections under the skin or into the muscles. It is more reliably absorbed with the injections.
One major complication of methotrexate is the development of liver cirrhosis when the medication is given over a prolonged period of time (years). The risk of liver damage is higher in patients who also abuse alcohol or have morbid (severe) obesity. Generally, periodic liver biopsies are recommended for a patient who has received a cumulative (total) methotrexate dose of 1.5 grams and higher.
Other side effects of methotrexate include low white blood cell counts and inflammation of the lungs.
Methotrexate should not be used in pregnancy.
Cyclosporine (Sandimmune) is a potent immunosuppressant used in preventing organ rejection after transplantation, for example, of the liver. It also has been used to treat patients with severe ulcerative colitis and Crohn's disease. Because of the approval of infliximab (Remicade) for treating severe Crohn's disease, cyclosporine probably will be used primarily in severe ulcerative colitis. Cyclosporine is useful in fulminant ulcerative colitis and in severely ill patients who are not responding to systemic corticosteroids. Administered intravenously, cyclosporine can be very effective in rapidly controlling severe colitis and avoiding or delaying surgery.
Cyclosporine also is available as an oral medication, but the relapse rate with oral cyclosporine is high. Therefore, cyclosporine seems most useful when administered intravenously in acute situations.
Side effects of cyclosporine include high blood pressure, impairment of kidney function, and tingling sensations in the extremities. More serious side effects include anaphylactic shock and seizures.
Infliximab (Remicade) is an antibody that attaches to a protein called tumor necrosis factor-alpha (TNF-alpha). TNF-alpha is one of the proteins produced by immune cells during activation of the immune system. TNF-alpha, in turn, stimulates other cells of the immune system to produce and release other proteins that promote inflammation. In Crohn's disease and in ulcerative colitis, there is continued production of TNF-alpha as part of the immune activation. Infliximab, by attaching to TNF-alpha, blocks its activity and in so doing decreases the inflammation.
Infliximab, an antibody to TNF-alpha, is produced by the immune system of mice after the mice are injected with human TNF-alpha. The mouse antibody then is modified to make it look more like a human antibody, and this modified antibody is infliximab. Such modifications are necessary to decrease the likelihood of allergic reactions when the antibody is administered to humans. Infliximab is given by intravenous infusion over two hours. Patients are monitored throughout the infusion for side effects.
Infliximab has been used effectively for many years for the treatment of moderate to severe Crohn's disease that was not responding to corticosteroids or immuno-modulators. In Crohn's disease patients, a majority experienced improvement in their disease after one infusion of infliximab. Some patients noticed improvement in symptoms within days of the infusion. Most patients experienced improvement within two weeks. In patients who respond to infliximab, the improvements in symptoms can be dramatic. Moreover, there can be impressively rapid healing of the ulcers and the inflammation in the intestines after just one infusion.
Only over the last few years infliximab also has been used to treat severe UCs. In a study of over 700 patients with moderate to severe UC, for example, infliximab was found to be more effective than placebo in inducing and maintaining remission.
Infliximab is typically given to induce remission in three doses - at time zero and then two weeks and four weeks later. After remission is attained, maintenance doses can be given every other month.
Infliximab, generally, is well tolerated. There have been rare reports of side effects during infusions, including chest pain, shortness of breath, and nausea. These effects usually resolve spontaneously within minutes if the infusion is stopped. Other commonly reported side effects include headache and upper respiratory tract infection.
Infliximab, like immuno-modulators, increases the risk for infection. One case of salmonella colitis and several cases of pneumonia have been reported with the use of infliximab. There also have been cases of tuberculosis (TB) reported after the use of infliximab.
Because infliximab is partly a mouse protein, it may induce an immune reaction when given to humans, especially with repeated infusions. In addition to the side effects that occur while the infusion is being given, patients also may develop a "delayed allergic reaction" that occurs 7-10 days after receiving the infliximab. This type of reaction may cause flu-like symptoms with fever, joint pain and swelling, and a worsening of Crohn's disease symptoms. It can be serious, and if it occurs, a physician should be contacted. Paradoxically, those patients who have more frequent infusions of Remicade are less likely to develop this type of delayed reaction compared to those patients who receive infusions separated by long intervals (6-12 months).
There are some reports of worsening heart disease in patients who have received Remicade. The precise mechanism and role of infliximab in the development of this side effect is unclear. As a precaution, individuals with heart disease should inform their physician of this condition before receiving infliximab.
There have been rare reports of nerve damage such as optic neuritis (inflammation of the nerve of the eye) and motor neuropathy (damage to the nerves controlling muscles).
There have also been rare reports of patients developing viral colitis (cytomegalovirus and herpes simplex virus) while on immunosuppressive medications. These viral infections can mimic a flare of ulcerative colitis and mistakenly suggest resistance to therapy. Before increasing the dose or changing the medication being used to treat the ulcerative colitis, patients should have a thorough evaluation including flexible sigmoidoscopy or limited colonoscopy with biopsies to help make the diagnosis of viral colitis.
Infliximab can aggravate and cause the spread of an existing infection. Therefore, it should not be given to patients with pneumonia, urinary tract infection or abscess (localized collection of pus).
It now is recommended that patients be tested for TB prior to receiving infliximab. Patients who previously had TB should inform their physician of this before they receive infliximab.
Infliximab can cause the spread of cancer cells; therefore, it should not be given to patients with cancer.
The effects of infliximab on the fetus are not known although the literature suggests that this medication is safe for women to continue until week 32 of pregnancy. At that time, the risk of exposure of the fetus to this medication by placental transfer is increased. Infliximab is listed as a pregnancy category B drug by the FDA (meaning there has been no documented human toxicity).
Because infliximab is partly a mouse protein, some patients can develop antibodies against infliximab with repeated infusions. The development of these antibodies can decrease the effectiveness of the drug. The chances of developing these antibodies can be decreased by concomitant use of 6-MP and corticosteroids. There are ongoing studies in patients who have lost their initial response to infliximab to determine whether measurement of antibody titers will be helpful in guiding further treatment. Results of these studies are not yet available.
While infliximab represents an exciting new class of medications in the fight against Crohn's disease and ulcerative colitis, caution is warranted because of potentially serious side effects. Doctors are using infliximab in moderate to severe ulcerative colitis not responding to other medications.
Adalimumab is an anti-TNF drug similar to infliximab. It decreases inflammation by blocking tumor necrosis factor (TNF-alpha). In contrast to infliximab, adalimumab is a fully humanized anti-TNF antibody containing no mouse protein and, therefore, might cause less of an immune reaction. Adalimumab is administered subcutaneously (under the skin) instead of intravenously as in the case of infliximab.
Rheumatologists have been using adalimumab for treating inflammation of the joints in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. It was also approved by the FDA in 2007 for the treatment of moderately to severely active Crohn's disease. Though not approved formally by the FDA for treatment of ulcerative colitis, a few studies have shown it to have some efficacy in treating patients with ulcerative colitis who are refractory to or have lost their response to infliximab. More information will be required before recommending this as a standard therapy.
Visilizumab is a humanized antibody that specifically binds to human CD3 expressing T cells, that inhibits the activity of the cells. (CD3 expressing T cells are part of the immune system and seem to play an important role in promoting the inflammation of ulcerative colitis.). In a phase 1 open-label study evaluating the safety and tolerability of this medication, 32 subjects received visilizumab. Results showed that 84% of these patients achieved a clinical response by day 30, 41% achieved clinical remission, and 44% achieved endoscopic remission. Main side effects were decreased CD4 counts and cytokine release syndromes (flu-like symptoms, etc), though there were no serious infections. Initial data seems promising though more must be learned about this medication before it can be used routinely. This medication is not yet approved by the FDA for treatment of ulcerative colitis.
Alpha-4 integrins on the surface of cells of the immune system help the cells to leave the blood and travel into the tissues where they promote inflammation. Antibodies against these integrins have been developed, to dampen the inflammatory response. Natalizumab is one such agent, and in small studies in patients with ulcerative colitis has been shown to have some efficacy in leading to clinical remission. Another more gut-selective humanized antibody (MLN02) has been evaluated in multi-center trials and has also been found to lead to clinical and endoscopic remission in more patients than placebo. More studies must be conducted to evaluate long term effectiveness and side effects of these medications. This medication is not yet approved by the FDA for treatment of ulcerative colitis.
Surgery for ulcerative colitis usually involves removing the entire colon and the rectum. Removal of the colon and rectum is the only permanent cure for ulcerative colitis. This procedure also eliminates the risk of developing colon cancer. Surgery in ulcerative colitis is reserved for the following patients:
Standard surgery involves the removal of the entire colon, including the rectum. A small opening is made in the abdominal wall and the end of the small intestine is attached to the skin of the abdomen to form an ileostomy. Stool collects in a bag that is attached over the ileostomy. Recent improvements in the construction of ileostomies have allowed for continent ileostomies. A continent ileostomy is a pouch created from the intestine. The pouch serves as a reservoir similar to a rectum, and is emptied on a regular basis with a small tube. Patients with continent ileostomies do not need to wear collecting bags.
More recently, a surgery has been developed which allows stool to be passed normally through the anus. In an ileo-anal anastomosis, the large intestine is removed and the small intestine is attached just above the anus. Only the diseased lining of the anus is removed and the muscles of the anus remain intact. In this "pull-through" procedure, the normal route of stool elimination is maintained. This procedure has a relatively good success rate, although pouchitis (inflammation of the distal ileum now acting as the rectum) is a well known complication (that should be confirmed by endoscopy) that is manifested by increased diarrhea, urgency, bleeding, and pain.
For refractory cases, oral steroids or IV infliximab can be used (though this is less well studied in distal colitis)
Also, surgery is recommended for severe colitis refractory to medical therapy
Although it seems plausible that a specialized diet might benefit patients with ulcerative colitis, there is actually no evidence to support treatment with dietary modification. Despite extensive research, no diet has been found to slow progression, treat, or cure the disease. It is recommended that patients stay on a balanced, healthy diet rich in fruits, vegetables, grains, lean meats, beans, fish, eggs, nuts. Patients should also try to limit foods with saturated fats high cholesterol. During flare-ups, patients should continue to eat as tolerated. The Crohn's and Colitis Foundation of America recommends a bland diet with soft food during a flare including hot cereals, boiled eggs, mashed potatoes, steamed vegetables, canned or cooked vegetables to minimize discomfort.
Active research is also ongoing to find other biological agents that are potentially more effective with fewer side effects in treating ulcerative colitis including adalimumab, visilizumab, and alpha-4 integrin blockers.
Research in ulcerative colitis is very active, and many questions remain to be answered. The cause, mechanism of inflammation, and optimal treatments have yet to be defined. Researchers have recently identified genetic differences among patients which may allow them to select certain subgroups of patients with ulcerative colitis who may respond differently to medications. Newer and safer medications are being developed. Improvements in surgical procedures to make them safer and more effective continue to emerge.
It is recommended that adults with inflammatory bowel disease generally follow the same vaccination schedules as the general population.
Osteoporosis has also increasingly been recognized as a significant health problem in patients with IBD. IBD patients tend to have markedly reduced bone mineral densities. Screening with a bone density study is recommended in:
For this reason, most patients with IBD should be on calcium and vitamin D supplementation.
 Ako niektoré vírusy spôsobujú rakovinu
Väčšinou sú účinky vírusu krátkodobé a relatívne mierne, ako bežné prechladnutie. Niekedy však tieto mikroorganizmy môžu spôsobiť vážnejšie škody a dlhodobé účinky určitých vírusov môžu zahŕňať kompli
Ako niektoré vírusy spôsobujú rakovinu
Väčšinou sú účinky vírusu krátkodobé a relatívne mierne, ako bežné prechladnutie. Niekedy však tieto mikroorganizmy môžu spôsobiť vážnejšie škody a dlhodobé účinky určitých vírusov môžu zahŕňať kompli
 Čo je to biliárna endoskopická sfinkterotómia?
Čo je to biliárna endoskopická sfinkterotómia? Sfinkterotómia je komplexný postup, ktorý zahŕňa použitie endoskopu (dlhá, tenká, flexibilná trubica, ktorá má na jednom konci svetlo a kameru) a zobra
Čo je to biliárna endoskopická sfinkterotómia?
Čo je to biliárna endoskopická sfinkterotómia? Sfinkterotómia je komplexný postup, ktorý zahŕňa použitie endoskopu (dlhá, tenká, flexibilná trubica, ktorá má na jednom konci svetlo a kameru) a zobra
 50 000 lajkov na Facebooku! Oslávme so skutočným jedlom!
Je čas na párty!! Čoskoro dosiahneme ďalší vzrušujúci míľnik a je čas oslavovať. Áno, máme párty a hádajte čo?! Všetci ste pozvaní! Minulú jar sme dosiahli hranicu 20 000 hodnotení Páči sa mi na Fa
50 000 lajkov na Facebooku! Oslávme so skutočným jedlom!
Je čas na párty!! Čoskoro dosiahneme ďalší vzrušujúci míľnik a je čas oslavovať. Áno, máme párty a hádajte čo?! Všetci ste pozvaní! Minulú jar sme dosiahli hranicu 20 000 hodnotení Páči sa mi na Fa